Studies of ocean circulation, which were carried out later in 19th century, involved investigation the distribution of salinity. Attempts were made to measure salt content by heating to remove the water from the sample by evaporation. Simple drying was accompanied by losses of violatile compounds and the hydroscopic nature of the thick residue made the measurement of its weight very difficult. A dry residue method was offered as a solution; the seawater sample was evaporated and dried to a stable weight at 480° C after processing with hydrochloric acid. On this basis salinity was defined as “the total amount of solid material in grams contained in one kilogram of seawater when all the carbonate has been converted to oxide, all the bromine and iodine replaced by chlorine, and all the organic material oxidized”.

Accordingly there was a need for a better method of determination total dissolved salts then the tedious and unreliable one of evaporating a sample. In 1889 the International Council for the Exploration of the Sea named Martin Knudsen (1871-1949) as chairman of a commission to study the problem of determination the salinity of seawater. Based on the premise of constancy of ionic ratios in seawater, the commission defined a “chlorinity” that could be determined by a simple volumetric titration using silver nitrate, to be used as a measure of salinity. Knudsen and his colleagues made measurement on samples of seawater from the different regions of the World Ocean and on the basis of comparation of nine determinations of salinity and chlorinity, proposed the formula: S = 0.03 + 1.805Cl (1)
which served oceanographers for the next 65 years. In his proposal Knudsen stressed the importance of the measurement of salinity of seawater in physical, climatological and biological investigations and mainteined that the measurement could be carried out by titration with an accuracy of 0.04 ppm but that was not generally achieved by the method them in use. Usually a few titrations were carried out by weighing and all volumetric titration were then referred to these. Knudsen pointed out that titration by weighing was at that time a fairly difficult operation and the errors in salinity determination were usually as high as 0.1-0.2 ppm. Obviously, better accuracy could be obtained in salinity determination if all water samples were examined in one laboratory but Knudsen realized that this would not be very practical. Instead he proposed that all interested nations should contribute to the establishment of an institution for procuring standard water. This institution would prepare (and standartize in terms of its chlorinity) the standard water and distribute samples to interested laboratories, together with a statement of the physical and chemical qualities (properties) of standard.

(1861-1930)
Self-portrait
Knudsen’s proposal has been reported in some detail on the Stockholm Conference in 1899. Persuasive though his arguments were, Knudsen’s proposal was not accepted in its entirety by the Conference. Despite his doubts, Knudsen’s arguments for a standard water evidently found favour with the members of the Conference, as it shown by the following quotation from the hydrographical programme which they agreed upon. A footnote explains this further: … “By Standard water shall be understood samples of filtered seawater, the physical and chemical properties of which are known with all possible accuracy by analysis, and statements of which are sent to the different laboratories”. Thus, the need for standard water for use in all laboratories was established.
It should be stressed that preparation of standard seawater was nothing new for Knudsen. Prior to the Stockholm Conference he had made five different batches of such standards for use in Danish hydrographic work. About 80 tubes of standard seawater were prepared in April 1900 and was distributed to Russia, Sweden, Norway, Finland and Germany and was used for all Danish titrations until August 1902.
Central Laboratory from which standard samples would be send, refers to proposal by Fridtjof Nansen (1861-1930), one of the Norwegian delegates to the Stockholm Conference. In meantime a second preparatory conference had been held in Christiania (Oslo) in 1901 at which Knudsen presented a provisional report on determination of the constants of seawater and then compilation of the Hydrographical Tables. In the hydrographic programme the use of Knudsen’s Tables was directly prescribed. It was further prescribed that “The same standard seawater shall be employed in all cases for standardizing the solution used for chlorine determinations“.

The most important result of these conferences was the establishment of the Standard Sea-Water Service, directed by F.Nansen. This was originally under control of I.C.E.S. and later under that of the International Association of Physical Oceanographers (I.A.P.O). In the autumn of 1902 the Central Laboratory was opened, but unfortunately it had a relatively short life. In 1908 Nansen decided that he no longer wished to continue as director and it was decided to close down the Laboratory. The Council agreed that, whereas the further elaboration of special problems in future must be entrusted to the specialists of the various countries, there remained “practical charges, in which all the hydrographers are concerned , e.g., the preparation of normal (standard) water. It seems natural to hand over again to Docent M. Knudsen this task …” Knudsen agreed to direct, on behalf of the Council, the preparation and distribution of the standard seawater were transferred from the Central Laboratory to Copenhagen in September 1908, where it remained until it was transferred to England in 1974.
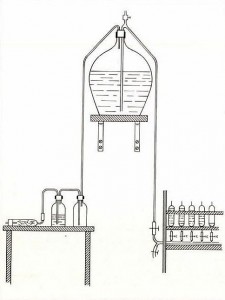
From the start of preparation of the standard has remained the same in principle though there have been some changes in detail. The water has usually been collected at the surface in the North Atlantic and transported in glass carboys or, more recently, polythene containers to the Standard Seawater Service premises. It is then pumped through filters into the storage tank and circulated through the filters for 2-3 weeks to achieve thorough mixing. During this period the seawater is gradually diluted with distilled water to give a final salinity near 35. For sealing the seawater in the glass ampoules the method used during the 20th century was similar to the one described by Knudsen in 1903.





 Visit Today : 266
Visit Today : 266 Visit Yesterday : 313
Visit Yesterday : 313 This Month : 6852
This Month : 6852 Total Visit : 318713
Total Visit : 318713 Hits Today : 350
Hits Today : 350 Total Hits : 738661
Total Hits : 738661 Who's Online : 1
Who's Online : 1